Dendrite "Tree" Growth
Certainly, the previous copper wire
experiment can be run on a comparative basis in which a first cell
would be dosed with a suitable quantity of the ion selective screening substance
and a second be used as a control.
The appearance of the two copper electrodes at the conclusion
of the experiment would reflect the effect of the ion selective screening substance;
with the dosed copper having acquired a hazy gray coloration
and the control copper having become darker.
However, the experiment takes in the order of 24 hours to complete
and the distinction between the two electrodes might not necessarily
appear clear cut via the camera. It would therefore be useful to
find a way of compressing the experiment's duration to, say, about
4 hours and also to use more convenient materials and simpler test
equipment.
In this regard tin appears to be able to provide an ideal substitute
for lead for experimental purposes, also being a group IV element,
having the same oxidation states of +2 and +4, possessing almost
identical electrochemical potentials at Pb + 2e + 2e 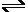 Pb -0.1262
V and Sn Pb -0.1262
V and Sn + 2e + 2e 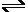 Sn -0.1375 V and having similar ionic radii. Both have high hydrogen
overpotentials. Tin is easy to handle and is relatively soluble
in sulfuric battery acid and can therefore help to provide an
excellent perspective on the actual electroplating process within
a comparatively short period of time. Sn -0.1375 V and having similar ionic radii. Both have high hydrogen
overpotentials. Tin is easy to handle and is relatively soluble
in sulfuric battery acid and can therefore help to provide an
excellent perspective on the actual electroplating process within
a comparatively short period of time.
This movie shows a positive electrode consisting of a coil of
97:3 tin-silver solder wire being electroplated onto a negative
electrode wire in regular sulfuric battery acid over a period
of 8 hours, although 4 hours would have been sufficient. All the
metal that can be seen growing on the negative wire originates
from the positive. It is much easier to monitor this growth than
to measure erosion at the positive electrode.
The extra time provides a magnified representation
of the interesting fractal dendritic development typical of plain
acid electroplating, closely resembling mossing sometimes found
in lead-acid battery cells, for example, after a period of 5 years.
(In commercial electroplating a smooth surface can only be obtained
by including metal salts and grain refiners in the electrolyte.)
TEST EQUIPMENT
Regular 12V automotive battery, for use as power supply;
150 ohm, 2 watt resistor, as current regulator. |

